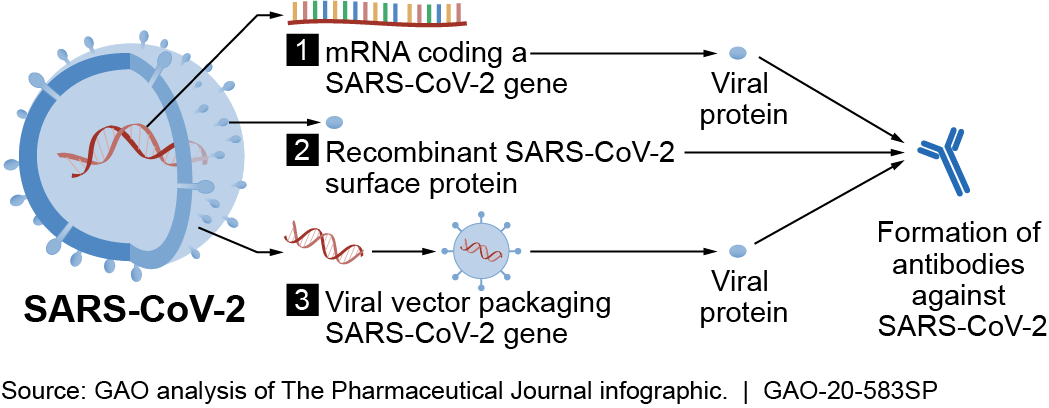
Developing Covid-19 Vaccines at Pandemic Speed An ideal vaccine platform would support development from viral sequencing to clinical trials in less than 16 weeks demonstrate elicitation of. An experimental vaccine is first tested in animals to evaluate its safety and potential to prevent disease.
 Science Tech Spotlight Covid 19 Vaccine Development
Science Tech Spotlight Covid 19 Vaccine Development
Standard vaccine development is a long process and studies are done in sequential steps.

Vaccine development process. The final stage in vaccine manufacture before distribution is fill and finish which is the process of filling vials with vaccines and packaging them for distribution. But it is important to note that no steps in this process were skipped. They perform studies to determinate a suitable formulation that can keep vaccine components stable to the end of its shelf life.
We invest in scientific and technical excellence to develop and launch a pipeline of new vaccines that meet the needs of patients and payers. Each vaccine under development must first undergo screenings and evaluations to determine which antigen should be used to invoke an immune response. This preclinical phase is done without testing on humans.
Companies first make small batches and do small scale studies to characterise and optimise the production process. Scientific principles in the development of the vaccine must follow the standard procedures be open and transparent and involve several experts. Throughout the vaccine development process scientists policymakers and.
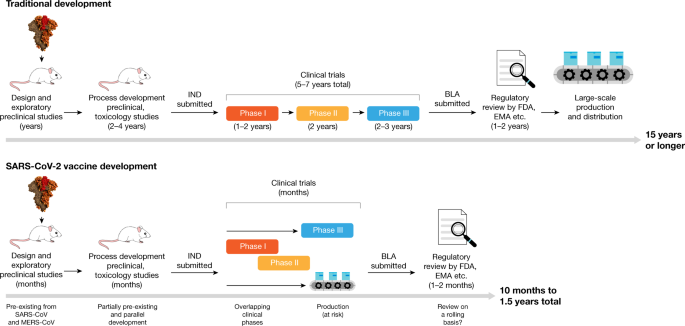
The manufacture of the vaccine must also be completed in the building that would be used to make the final product. Estimated the cost of early development and initial clinical safety trials for a typical vaccine to be in the range of 3168 million. The development of the COVID-19 vaccine was unique in that stages were permitted to proceed simultaneously.
Vaccine development is a complex multistep process that includes rigorous clinical testing and regulatory hurdles. The COVID-19 vaccines were not rushed but were prioritized. In total a vaccine can take more than 10 years to fully develop and costs up to 500 million the UK charity says.
The two vaccines rolling out across the country since late December have made the vaccine development and production process look easy. Such technologies have helped vaccine manufacturers achieve consistent product purity and quality rapidly and cost effectively. Dont vaccines usually take longer than this.
This is the normal timeline for vaccine development. This is the period of time where scientists attempt to determine if they can identify a part of the virus which stimulates the immune memory so that the immune system can quickly destroy the pathogen before it can do harm. The scientific principles and procedures must be applied to demonstrate that the production of vaccines has prioritized the principles of prudence and can be accounted for scientifically he emphasized.
The typical vaccine development process. Discovery using the isolated pathogen to develop a possible vaccine. Typical vaccine development process starting in the lab through post-FDA-approval monitoring COVID-19 Vaccine Safety Surveillance Ongoing FDA monitoring of COVID-19 vaccine safety.
Figure 1Vaccine process development general approach Scientists have made significant breakthroughs in bioprocess and analytical technologies for supporting vaccine development. Although this is a conceptually simple part of the vaccine manufacture process it is often a bottleneck in the process of distributing and administering vaccines. Vaccine development is characterised by a high failure rate often 93 between animal studies and registration of a product The discovery and research phase is normally two-to-five years according to the Wellcome Trust.
Large scale trials to determine the efficacy of a vaccine. The company must continue to keep the FDA apprised of its progress and results during this time. Our work to research develop and introduce a new vaccine typically goes through several stages.
Learn more about how the vaccine was developed and approved below. FDAs Center for Biologics Evaluation and Research CBER ensures that FDAs rigorous scientific. At any time during this process the company or the FDA can decide against continued development.
Ensuring the safety and effectiveness of vaccines is one of FDAs top priorities.
 Advances In Purification Technologies Accelerate Vaccine Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
Advances In Purification Technologies Accelerate Vaccine Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
 Sars Cov 2 Vaccines In Development Nature
Sars Cov 2 Vaccines In Development Nature
 Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
 Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency
Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency
 The Covid 19 Vaccine Development Multiverse Nejm
The Covid 19 Vaccine Development Multiverse Nejm
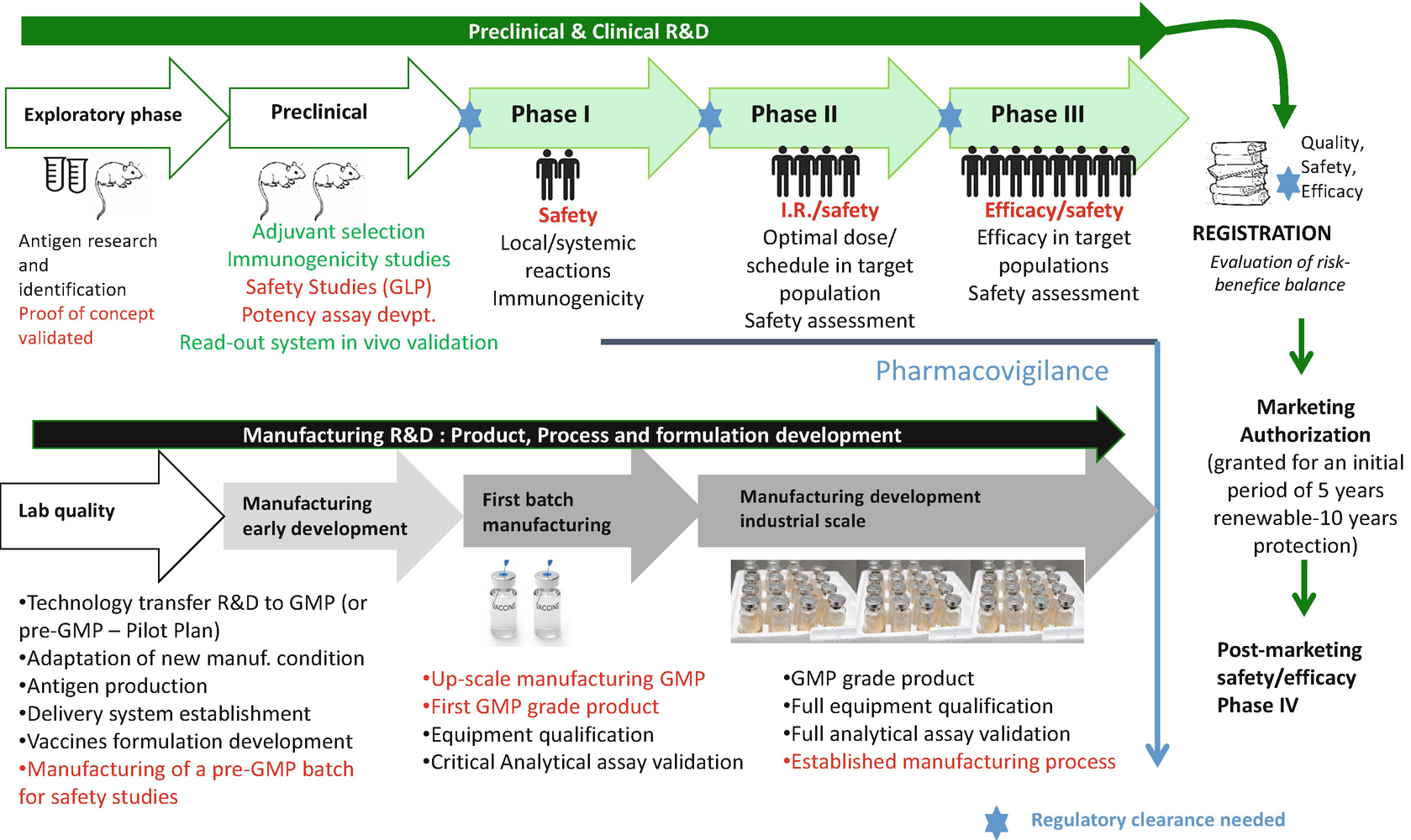 Vaccine Development From Preclinical Studies To Phase 1 2 Clinical Trials Springerlink
Vaccine Development From Preclinical Studies To Phase 1 2 Clinical Trials Springerlink
Https Www Who Int Immunization Policy Who Vaccine Development Policy Pdf
 Developing Covid 19 Vaccines At Pandemic Speed Nejm
Developing Covid 19 Vaccines At Pandemic Speed Nejm
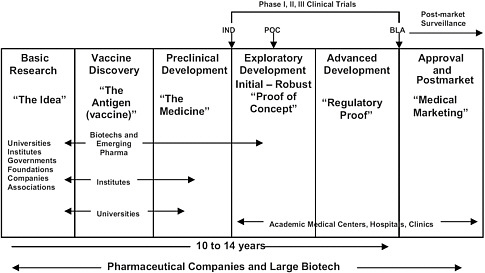 5 New Vaccine Development And The Future Needs Of The Special Immunizations Program Protecting The Frontline In Biodefense Research The Special Immunizations Program The National Academies Press
5 New Vaccine Development And The Future Needs Of The Special Immunizations Program Protecting The Frontline In Biodefense Research The Special Immunizations Program The National Academies Press
 Producing Prevention The Complex Development Of Vaccines Resources
Producing Prevention The Complex Development Of Vaccines Resources
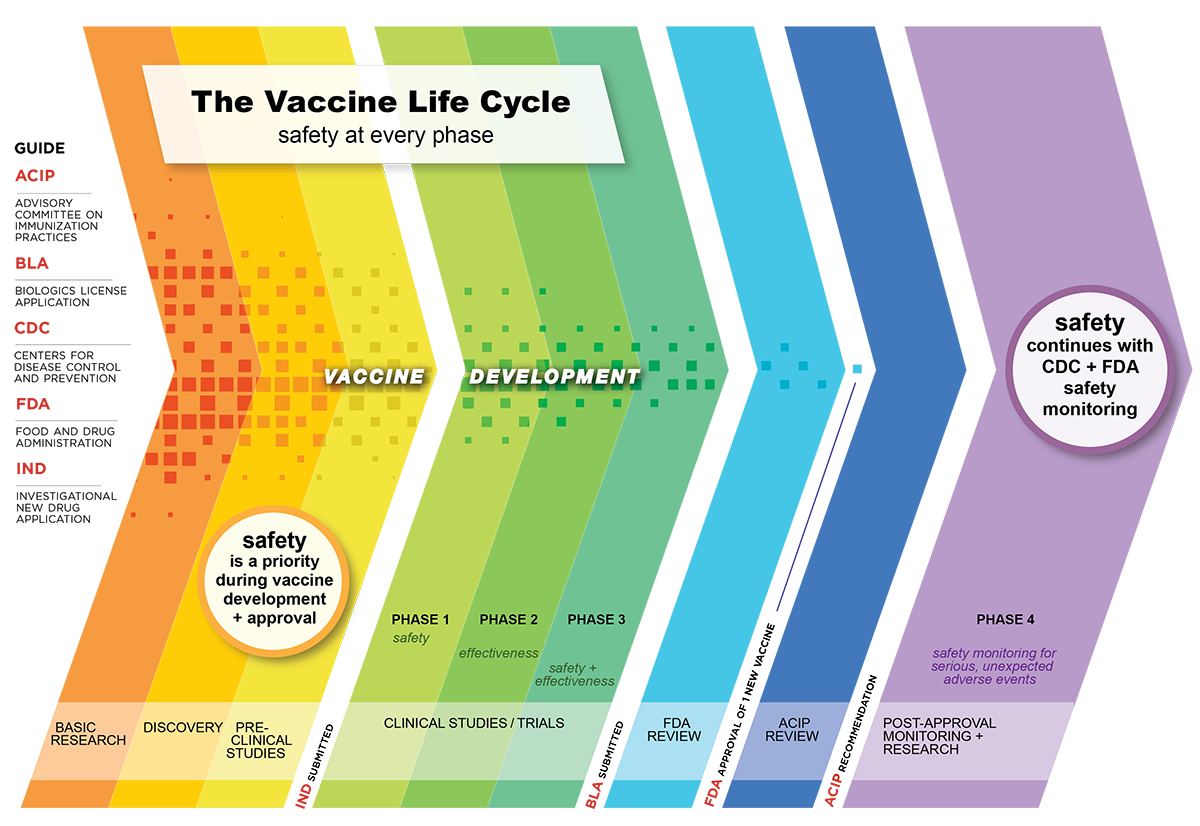 U S Vaccine Safety Overview History And How It Works Cdc
U S Vaccine Safety Overview History And How It Works Cdc
 The Process Of Designing And Testing A Vaccine Bactivax
The Process Of Designing And Testing A Vaccine Bactivax
 Vaccine Development Past Present And Future Bmg Labtech
Vaccine Development Past Present And Future Bmg Labtech
No comments:
Post a Comment
Note: only a member of this blog may post a comment.